Introduction
Internal sizing develops resistance to penetration of aqueous liquids throughout the sheet. Internal sizing is accomplished by adding materials to the stock before the headbox to retard water penetration into the final paper. Water penetration is retarded by the nonpolar portions of the size molecule. A reactive portion of the size molecule anchors it to the surface of the fiber.
Surface sizing works by a different mechanism and occurs at the size press where an application of starch (or other material) fills the capillaries of paper, making water penetration much more difficult. Starch is not hydrophobic as are internal sizing agents.
Rosin sizing with alum, used since the early 1800s, is only effective below pH of 7 or so. Alkaline sizing was developed in the 1940s and 1950s. It has recently enjoyed widespread use in the United States in printing papers filled with calcium carbonate that must be used at pH of 7 or higher because in acid conditions it decomposes to carbon dioxide gas that causes pitting of the sheet.
Paper may be:
- Hard-sized (high resistance to liquid penetration, such as many printing and packaging papers),
- Slack-sized (low resistance to liquid penetration, such as newsprint), or
- No-sized (toweling, blotting, and sanitary papers).
There are two common methods used in internal sizing:
- Rosin sizing, which must be carried out at pH 4–6,
- Alkaline sizing, carried out at pH 8 or higher with alkenyl succinic anhydride (ASA) or alkyl ketene dimer (AKD).
Advantages of alkaline papermaking include:
- Longevity due to absence of residual acid,
- Ability to use calcium carbonate (a bright and inexpensive filler),
- Stronger and less brittle paper,
- Less corrosion on the paper machine,
- Fewer issues with recycled fiber containing calcium carbonate.
Hardwood pulps are much easier to size than softwood pulps. Sulfate pulps are easier to size than sulfite pulps, thermomechanical pulps, or groundwood pulps. The explanation centers around the number of carboxylate groups as anchor points in sizing.
Internal Sizing with Rosin
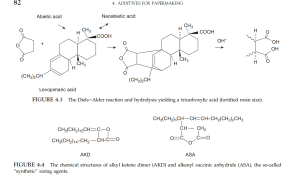
The most common internal size traditionally is rosin (2–9 kg/t or 4–20 lb/ton), precipitated with alum (Al₂(SO₄)₃), a process developed in 1807. Abietic acid and its homologues are fortified by maleic anhydride addition via the Diels-Alder reaction.
Rosin may be used in:
- Salt form (soap size, pH 10–11),
- Paste (solid salt with 20–30% water),
- Free acid form (emulsion size), effective at slightly higher pH.
Sizing sequence:
- Normally, rosin is added before alum.
- If alum is added first, it’s called reverse sizing, useful in high-calcium water or alkaline systems.
Internal Sizing with ASA or AKD
Papers made under alkaline conditions use synthetic sizing agents such as:
- AKD (e.g., Aquapel) – prepared via dimerization of fatty acid acyl chlorides,
- ASA – made by reacting C-16 to C-20 olefins with maleic anhydride.
 Important Notes:
Important Notes:
- ASA must not hydrolyze with water to remain effective.
- Emulsions of ASA should be stored short-term under acidic conditions (pH 3.5–4).
- AKD is more stable and can be stored as a dilute emulsion for weeks to 3 months.
- Both must be emulsified with cationic starch.
Emulsification guidelines:
- ASA: starch cooled <38°C, pH 4–4.5,
- AKD: starch solution pH 5–7, intrinsic viscosity 1.0–1.1 for max sizing.
Synthetic sizes may include cationic groups to improve retention. ASA is typically used with 4–5 kg/t alum and cures quickly on-machine. AKD may be inhibited by alum and requires 1–2 weeks off-machine to fully cure.
Challenges in mills using synthetic sizes:
- Slippery paper (low coefficient of friction),
- Reduced forming fabric life,
- Increased picking at press section,
- Formation issues in calcium carbonate-filled papers,
- Poorer sizing at the size press.