1. Synthesis of AKD
1.1 Preparation of Acid Chloride
The raw material for industrial-grade AKD (alkyl ketene dimer), commonly used in papermaking, is typically a mixture of saturated fatty acids. Acid chloride is produced by reacting these fatty acids with thionyl chloride, as shown in the following reaction:
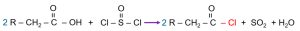
Since separating individual organic acids is extremely difficult—a single natural fat source may contain at least five different acids, and sometimes as many as twelve—the raw material used in papermaking is usually a mixture of stearic acid (C₁₈H₃₆O₂) homologs. The commonly referred to “1840” and “1865” waxes indicate stearic acid contents of 40% and 65%, respectively.
1.2 Formation of Ketene Intermediate
Acid chloride reacts with triethylamine, removing hydrogen chloride and forming an unstable ketene intermediate:
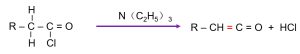
1.3 Dimerization via Intramolecular Lactone Formation
The unstable ketene undergoes intermolecular condensation to form a lactone ring, resulting in the final AKD molecule—hence the name “alkyl ketene dimer”:
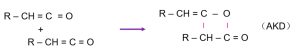
The molecular structure of AKD can also be expressed as:
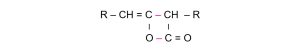
2. Emulsification of AKD
2.1 Emulsifier Preparation
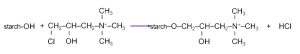
AKD emulsions are typically stabilized with cationic starch and polymeric emulsifiers. Cationic starch is produced by reacting tapioca starch with etherifying agents:
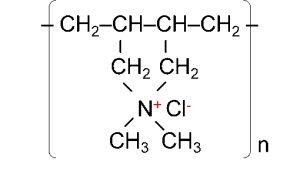
A common polymer emulsifier is PolyDADMAC (poly(diallyldimethylammonium chloride)):
2.2 AKD Emulsification Process
The emulsification of AKD is a physical dispersion process, where hydrophobic wax is dispersed into water to form a stable emulsion.
Key steps include:
-
Heating to melt AKD wax
-
Adding emulsifiers
-
Applying high-shear mixing and homogenization
-
Cooling the emulsion
-
Adding aluminum sulfate, stabilizers, and other additives
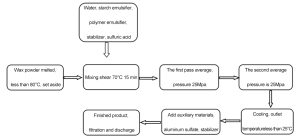
The result is a positively charged AKD emulsion
3. AKD Sizing
3.1 Sizing Mechanism
The hydrophobicity of sized paper comes from the chemical reaction between AKD and cellulose. AKD molecules consist of a hydrophobic alkyl chain and a hydrophilic lactone ring. Cellulose fibers are naturally hydrophilic due to numerous –OH groups. When AKD reacts with these groups, hydrophobic chains are anchored to the surface, making the paper water-resistant:
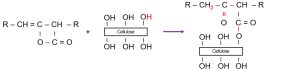
The reaction fixes one end of the AKD molecule onto the fiber while the alkyl chain points outward, forming a hydrophobic surface
3.2 Sizing Process
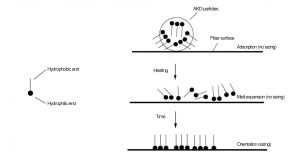
AKD sizing involves three main stages:
-
Adsorption: AKD particles adsorb onto fiber surfaces, similar to filler retention. This occurs in the forming section.
-
Melting and Diffusion: During drying, AKD melts (above its melting point) and diffuses into fibers.
-
Chemical Reaction: AKD reacts with –OH groups on cellulose, forming covalent ester bonds. This slow reaction is key to effective internal sizing.
4. Key Application Factors
4.1 pH and Alkalinity Control
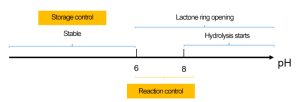
The sizing reaction depends on the lactone ring in AKD. It opens under neutral to alkaline conditions, reacting with fiber –OH groups to form esters. However, excessive pH accelerates hydrolysis, reducing sizing efficiency.
-
Optimal pH for reaction: 6.0–8.0
-
Recommended system pH: 7.5–8.5
-
Storage pH for AKD emulsion: 2.5–3.5
When pH exceeds 8, hydrolysis increases. pH below 6 hinders the sizing reaction but helps in storage stability.
Recommended alkalinity (as buffering capacity): 50–200 ppm
This helps maintain stable pH when acidic AKD emulsion is added to the system.
4.2 Temperature Control
Temperature influences both AKD emulsion stability and reaction efficiency:
-
AKD emulsions are thermodynamically unstable suspensions.
-
High temperature increases particle collision and aggregation risk.
-
Electrostatic repulsion from cationic particles helps maintain emulsion stability.
At elevated temperatures, AKD particles may skin or settle, but this is not necessarily due to hydrolysis. Since the hydrolysis is exothermic, higher temperatures may actually shift equilibrium toward ester formation.
Recommended values:
-
Storage temperature: 5–30°C
-
System temperature: 45–50°C
-
AKD melting point: ~45–55°C
-
Dryer section temperature: ≥60°C (for full reaction and diffusion)
To maximize retention and performance:
-
Add AKD near the headbox to avoid premature melting.
-
Ensure sufficient drying temperature and retention time.
5. Conclusion
Effective AKD sizing in neutral systems requires tight control of pH, alkalinity, and temperature throughout the process. Mastering these parameters ensures efficient sizing and high-quality paper. Challenges remain in application and formulation, requiring continued industry collaboration and innovation.